5 Ml of Hcl to Grams
8300 Ml This problem has been solved. Now for the mass in grams of HCl.
182 g of a metal require 325 ml of 1 N HCl to dissolve it.

. For Study plan details. How many mL of 0015M HCL are needed to titrate 50 grams of sodium hydroxide. There are 5 grams in 5 ml because 1 milliliter equals 1 gram.
If 137 mL of hydrochloric acid solution is taken then 137 mL x 118 gmL 162 g is the mass of the hydrochloric acid solution. 0 0 Similar questions. A 46 B 65 C 56 D 42 Easy Solution Verified by Toppr Correct option is C Gram equivalent weightW 1000NV Gram equivalent weight182 10001325 Gram equivalent weight 3251820 56 Was this answer helpful.
Usually we are ultimately interested in the number of moles of acid used. How many grams of HCl are needed to make a 15 molal solution in 10 kg of H 2 O. Mass of HCl present in the solution 250 3646.
The molecular weight of HCl is 3647 gmol. The SI base unit for amount of substance is the mole. This means that your sample will contain 1191g solution 37 g HCl 100g solution 44067 g HCl.
1 grams HCl is equal to 0027426610504282 mole. The mixture is. Calculate the pH of a solution of at 25C made by adding 285 g of HCl to 250 mL of H2O.
Calculate the pH of a solution of at 25C made by adding 285 g of HCl to 250 mL of H 2 O. We need to use the molar mass of calcium hydroxide along with the molar concentration of HCl to determine the volume in mL of HCl needed. 4 moles HCl to grams 14584376 grams.
Number of moles Concentration Volume. 6 moles HCl to grams 21876564 grams. 5 How much water would I need to add to 500 mL of a 24 M KCl solution to make a 10 M solution.
Starting early can help you score better. Students whove seen this question also like. Use the following chemical equations to assist determining the mass FeOH3 aq 3HCI aq FeClz aq 3H20 HClaq NaOH aq NaCl.
9321924448 1800-212-7858 9372462318. What is the equivalent weight of metal. 7 moles HCl to grams 25522658 grams.
Contact us on below numbers. The answer is 3646094. The amount of moles of HCl needed is found by using the formula MV moles where M molarity 0100M V volume in liters 1000mL1L so 5000mL 5000mL1L1000mL 0500L 01000500005mol convert 005moles of HCl to grams using the formula moles massmolar mass solving for mass gives mass molesmolar mass molar mass of HCl 36gmol.
1 moles HCl to grams 3646094 grams. Molecular weight of HCl or mol This compound is also known as Hydrochloric Acid. Check out a sample QA here.
To convert mass to moles we need the molecular weight. The mass of the sample you have here will thus be 1000mL 1191 g 1mL 1191 g Now you know that the solution has a 37 concentration by mass which means that every 100 g of solution will contain 37 g of hydrochloric acid. So if you want to convert ml of HCl into grams 5 x 12 is 6 grams.
Well the density of HCl is 12gml. 3 moles HCl to grams 10938282 grams. How many grams HCl in 1 mol.
Molecular mass of HCI 365 Molarity 05 M 05 Mol L-1 Volume of solution 250 cm3 So number of moles in 250 cm3 MV_L 05XX 2501000 054 0125 moles Weight of HCl dissolved number of moles of HCI xx 365 0125 xx 365 45625 g. Number of moles of HCl present 500 050. How do you convert 5 ml HCl to grams.
The molar mass of calcium hydroxide is 74093 grams per mole. Convert a 4 mgdL Mg 2 standard to mEqL units Mg 2430 g A 150 wv solution of CaCl 2 is needed. 8 moles HCl to grams 29168752 grams.
How many mL of 0015M HCL are needed to titrate 50 grams of sodium hydroxide. See the answer See the answer See the answer done loading. Well the density of HCl is 12gml.
1200 mL will be the final volume of the solution. US Fluid Ouncefl oz. Want to see the full answer.
Number of moles of HCl present 250 moles. You can view more details on each measurement unit. How many mL of 0015M HCL are needed to titrate 50 grams of sodium hydroxide.
Avail 25 off on study pack. Click hereto get an answer to your question 100 ml of 05 N NaOH solution is added to 10 ml of 3 N H2SO4 solution and 20 ml of 1 N HCl solution. 1 cubic meter of Hydrochloric acid weighs 16423 kilograms kg 1 cubic inch of Hydrochloric acid weighs 000094931 ounce oz Hydrochloric acid weighs 00016423 gram per cubic centimeter or 16423 kilogram per cubic meter ie.
There are 15 grams in 15 ml because 1 milliliter equals 1 gram. How much of the 950 vv EtOH should you use to prepare the 500-mL solution. 16423 kilograms kg of Hydrochloric acid fit into 1 cubic meter 000094931 ounce oz of Hydrochloric acid fits into 1 cubic inch Hydrochloric acid weighs 00016423 gram per cubic centimeter or 16423 kilogram per cubic meter ie.
At 0C 32F or 27315K at standard atmospheric pressure. How do you convert 5 ml HCl to grams. At 0C 32F or 27315K at standard atmospheric pressure.
2 moles HCl to grams 7292188 grams. In the stock room is a bottle of 950 vv EtOH. A sample of unknown mass of ironIlI hydroxide was added to 625 mL of 0280 M HCI The resulting acidic solution was titrated to an endpoint with 238 mL of 0113 M NaOH What mass of ironIlI hydroxide was added to the HcI.
We assume you are converting between grams HCl and mole. 5 moles HCl to grams 1823047 grams. Molar mass of HCl 3646 gmol.
So if you want to convert ml of HCl into grams 5 x 12 is 6 grams. Using the above example of 137 mL 162 g of hydrochloric acid solution. Density of hydrochloric acid is equal to 16423 kgm³.
Mass Number of moles Molar mass. Density of hydrochloric acid is equal to 16423 kgm³. The 5 ml to grams will not only find out 5 ml equals how many grams it will also convert 5 milliliter to other units such as quarts pint cup tablespoon teaspoon and.
Solution for How many grams of hydrogen chloride are in 500 mL of concentrated 120 M HCl solution. How Many Grams is 5ml.

Titrations Practice Worksheet Practices Worksheets Worksheets Worksheet Template
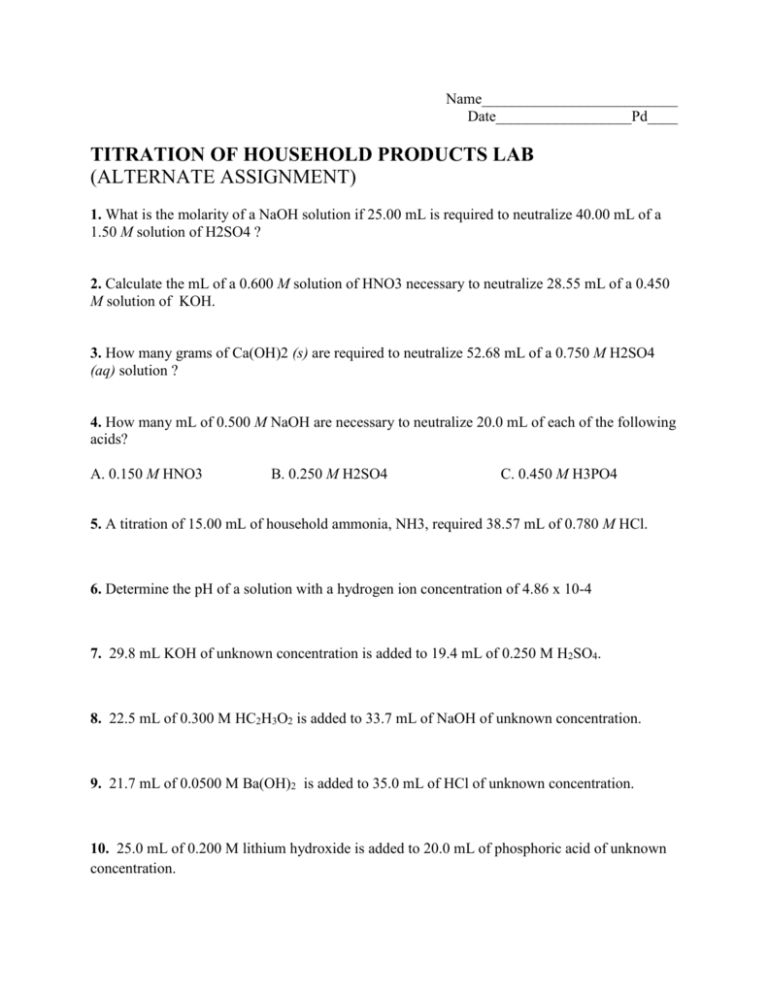
Titration Lab Alternate Assignment

Solved 3 Calculate The Moles And Grams Of Hcl Present In Chegg Com

5 Ml Of N Hcl 20 Ml Of N 2 H So And 30 Ml Of N 3 Hno Are Mixed Together Neetlab

Solved Student Yut The Solubility Of Potassium Chlorate Kcio At Various Evaluating Temperatures She Dissolves 2 50 G Of Kcios In A Total Volume Of7 5 Ml Of Water Calculate The Concentration Of Kcio

Calculate The Concentration Of Hcl Acid If 50 Ml Of Hcl Is Required To Neutralize 25 Ml Of 1 M Naoh In Acid Base Titration

How Many Moles Of Hcl Are Present In 1 Litre Of 1 M Hcl Solution Youtube

Molarity Worksheet Word Problem Worksheets Literal Equations Word Problem Practice
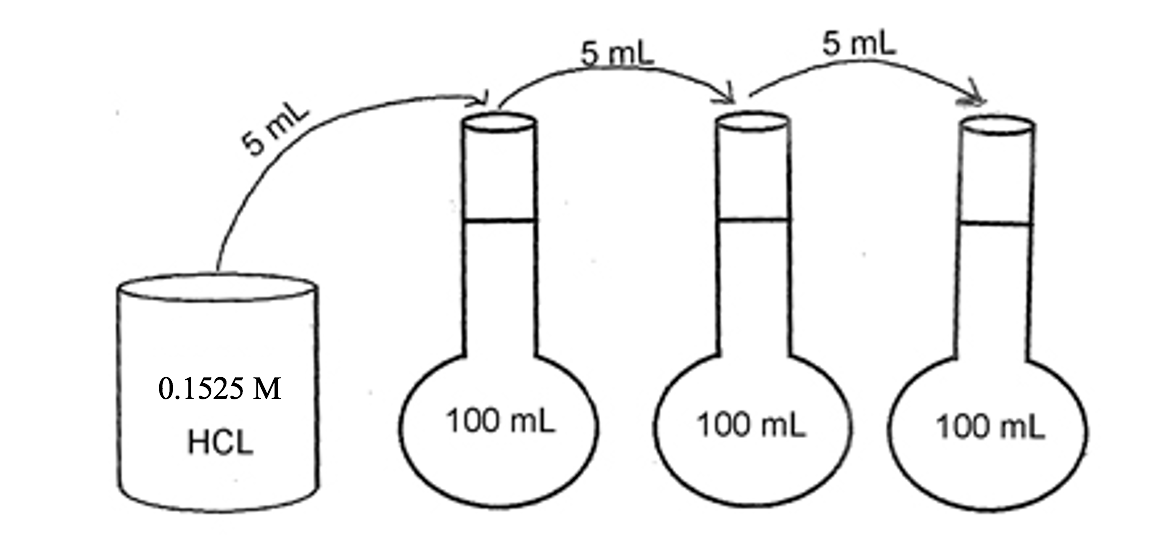
Solved Starting With A 0 1525 M Hcl Stock Solution Three Chegg Com
What Will Be The Resulting Solution When 100 Ml Of 1 0 M Hcl Is Mixed With 75 Ml Of 1 0 M Na2co3 Quora

Calculate Normality And Molarity Of The Following A 0 74 G Of Ca Oh 2 In 5 Ml Of Solu Youtube

Solved How Many Moles Of Hcl Are In 10 5 Ml Of A 12 M Hcl Chegg Com

Calculate The Mass Of Anhyrous Hci In 10ml Of Concentrated Hci Density 1 2 G M Youtube

50 0ml Of 0 10 M Hcl Is Mixed With 50 0ml Of 0 10 M Naoh The Solution Temperature Rises By 3 0 Youtube

Children S Benadryl Allergy Liquid Benadryl

Pin By Helen Gibson Dubeau On Eggs In 2022 Nutrition Healthy Eating Healthy Liver Healthy Eating Recipes

5 Ml Images Stock Photos Vectors Shutterstock

Pin By Chem Projects On Synthesis Of Caffeine From Uracil Cam Sutton And Nick Valle Methylation Beaker Solutions


Comments
Post a Comment